Scientific Data and Guidance
for inflammatory bowel diseases as an example for autoimmune diseases

Helminthic Therapy:
Scientific Data and Guidance for inflammatory bowel diseases as an example for autoimmune diseases
The inflammatory bowel diseases (IBD) are comprised of two major phenotypes, Crohn’s disease (CD) and ulcerative colitis (UC). It is likely that chronic inflammation in IBD is due to aggressive cellular immune responses to intestinal antigens which are not yet well characterized in genetically predisposed individuals. Standard therapy for the symptomatic treatment of IBD includes sulfasalazine, mesalazine, systemic and topical glucocorticoids, immunosuppressants like azathioprine or 6-mercaptopurine, and in severe cases also treatment with anti-TNF-α therapy, methotrexate, or cyclosporine as monotherapy or in combination with other agents.
Profound alterations in mucosal immunity have been demonstrated in patients with IBD. The cross-inhibitory Th1/Th2 balance is an important regulatory mechanism of the gastrointestinal immune system. The Th1/Th2 balance is regulated by the local cytokine environment within the intestinal mucosa. There is a reciprocal down-regulation of Th1 proliferation by Th2 cytokines and vice versa. Particularly in CD, there is strong evidence of a CD4 T helper cell type 1 (Th1) immune response that contributes to the pathogenesis of disease. Originally, the rationale for performing research with helminths was based on the hygiene hypothesis, which was refined from the “Old friends Hypothesis” for diseases resulting from disorders of the immune system, which postulates that multiple childhood exposures to parasites and pathogens protect an individual from allergic and autoimmune disease later in life, whereas individuals raised in a more sanitary environment are more likely to develop autoimmune diseases and allergies. In line with these hypotheses, epidemiologic evidence, case control observations, animal studies and clinical studies all suggest that exposure to helminth parasites which followed the whole human evolution since its early beginnings may afford protection from or even treat autoimmune disorders and also IBD.
Helminth infections shift the host’s immune bias from Th1 response to augmented Th2 responses. Typically, this helminth-induced response involves the production of the cytokines interleukin-4 (IL-4), IL-5, IL-9, IL-10, and IL-13, as well as immunoglobulin E (IgE) and the expansion and mobilization of specific effector cells, such as mast cells, eosinophils, and basophils. A stimulation of a Th2 response by a helminthic infection may suppress a predominant Th1 situation by the inherent cross-inhibitory mechanisms. However, it seems unlikely that helminths simply alter the Th1/Th2 balance. Recent studies indicate that helminths stimulate the development of regulatory T-helper cells (Treg) that reduce both Th1 and Th2 responsiveness.
Treg lymphocytes actively suppress the differentiation of both, Th1 and Th2 lymphocytes by the secretion of IL-10 and transforming growth factor-β (TGF-β).
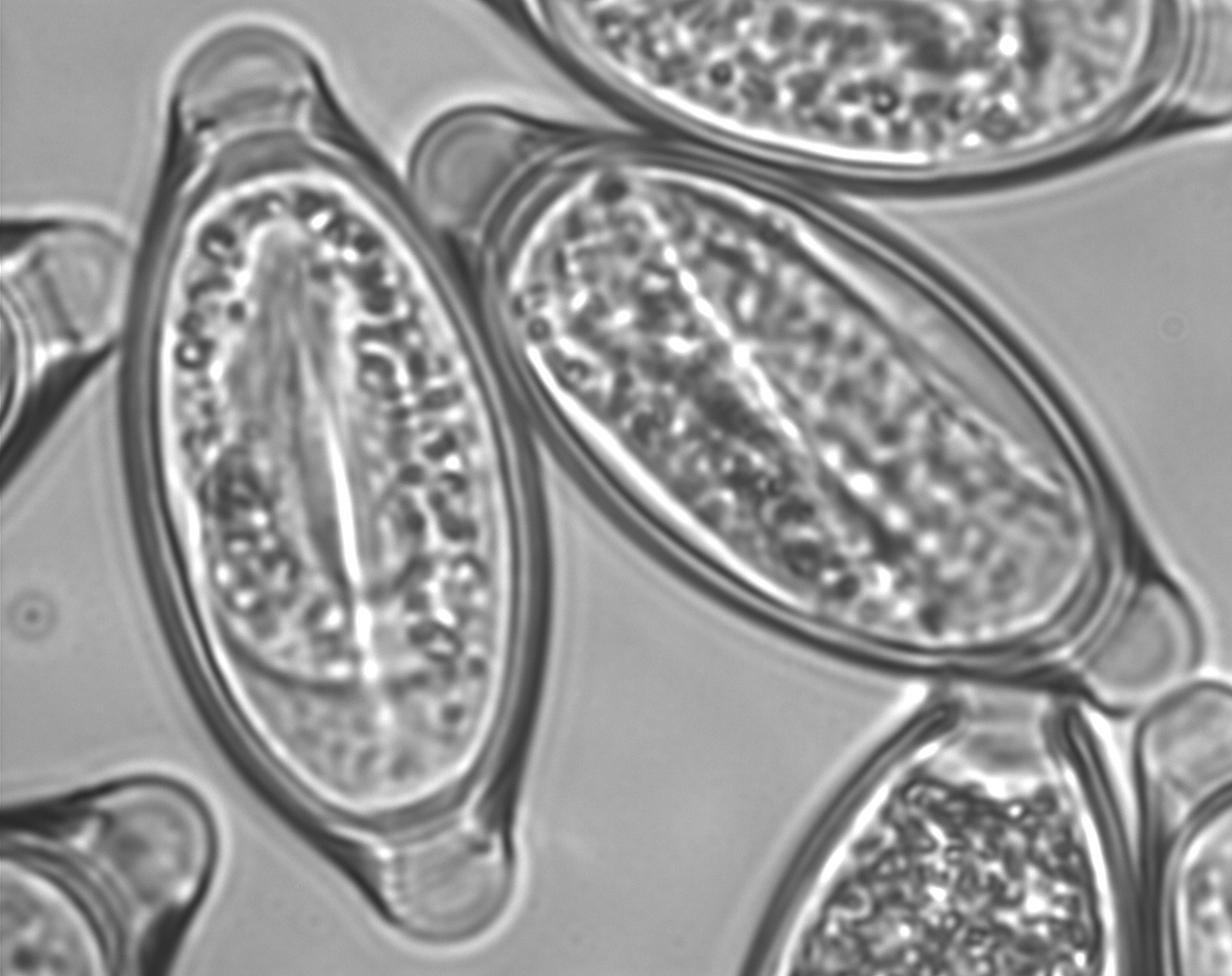
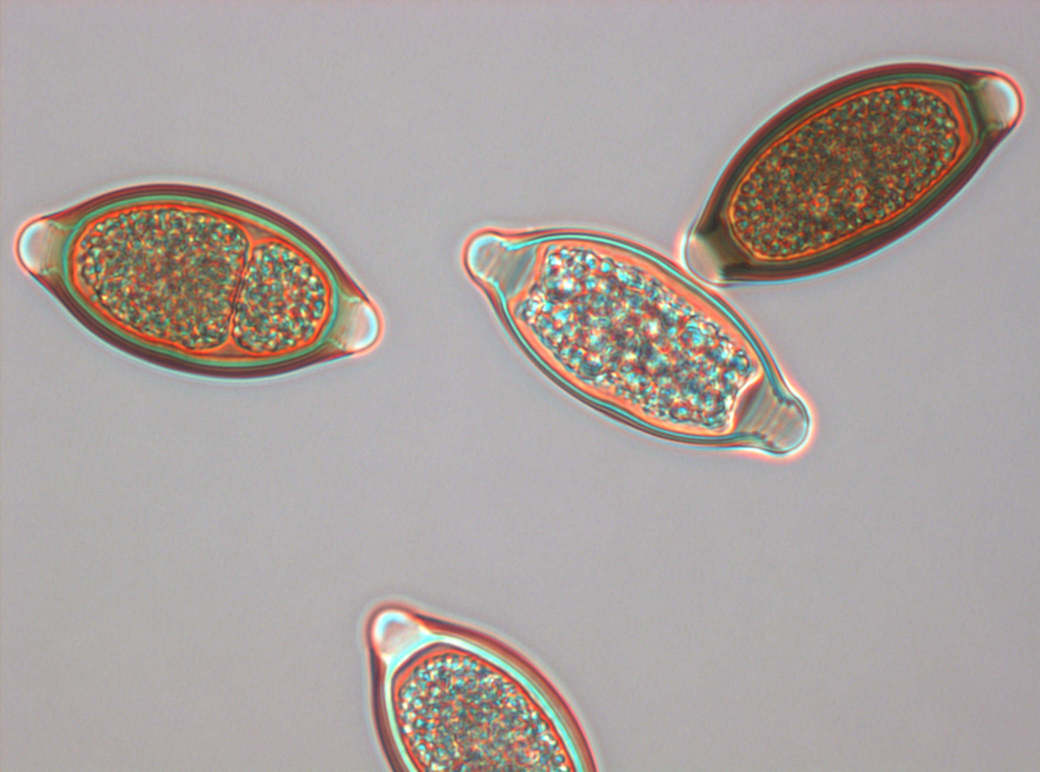
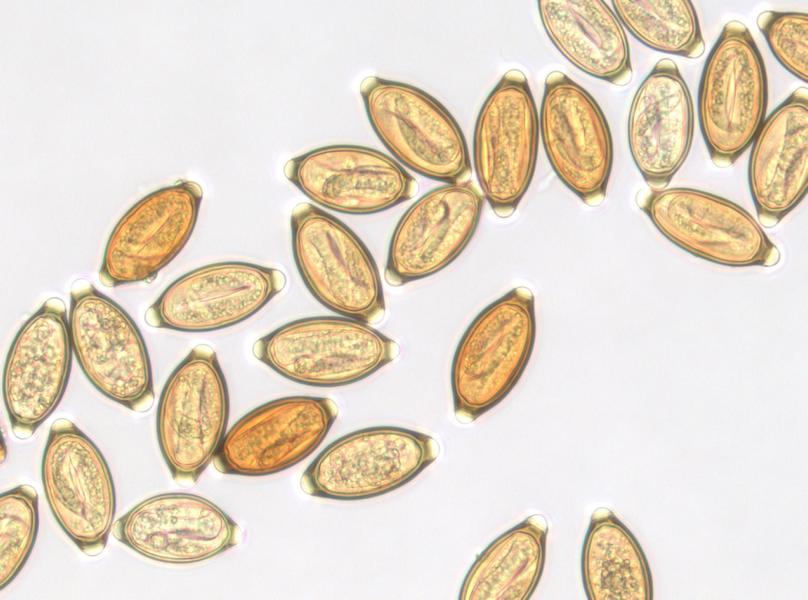
Early experiences suggest that embryonated eggs of the porcine whipworm Trichuris suis (Trichuris suis ova, TSO®) colonize the human intestine without invading or infecting.
Safety and efficacy of TSO® have been investigated in clinical studies with patients suffering from IBD, Multiple Sclerosis, allergies and other autoimmune diseases. The results of the previous trials indicate that Trichuris suis therapy might be effective in both forms of IBD, CD and UC. Results of treatment with a single dose were temporary in some patients, but it was possible to maintain a remission with a scheduled administration of TSO® every 2-3 weeks for longer periods.
The clinical trials in IBD demonstrated improvement of disease activity, with remission observed in many patients, i.e., TSO® has been shown to be effective not only in treating active disease, but also in maintaining remission. Treatment with TSO® appeared to be safe in all clinical trials.


Nature’s most powerful probiotic:
Introduction
The inflammatory bowel diseases are characterized by their inflammatory nature, their chronic remitting and relapsing course, and their unknown etiology. While research has led to great advances in understanding the inflammatory bowel diseases and their underlying pathophysiologic mechanisms, treatment options are still limited. Genetic, immunologic, and environmental factors play important roles in disease pathogenesis, but it is likely that chronic inflammation in IBD is due to aggressive cellular immune responses to intestinal antigens not yet determined in genetically predisposed individuals.
Helminthic Therapy:
Immune Dysregulation in IBD
Mucosal homeostasis is a balancing act between effector T cells (T helper cells type l and type 2, Th1/Th2) and regulatory T cells. An increase in the effector cell population with excessive inflammatory responses or a decreased function of regulatory T cells may result in mucosal inflammation.
Profound alterations in mucosal immunity have been demonstrated in patients with IBD. Particularly in CD, there is strong evidence of a CD4 T helper cell type 1 (Th1) immune response that contributes to the pathogenesis of disease. The Th1 response typically occurring in viral and bacterial infections is characterized by an increase in interleukin (IL)-12 and interferon-γ. In contrast, Th2 response is characterized by increased IL-4, IL-5, and IL-13. Polarized Th1 responses impede expression of Th2 cytokines and polarized Th2 responses impede Th1 activity.
The cross-inhibitory Th1/Th2 balance is an important regulatory mechanism of the
gastrointestinal immune system. The Th1/Th2 balance is regulated by the local cytokine environment within the intestinal mucosa. There is a reciprocal down-regulation of Th1 proliferation by Th2 cytokines and vice versa. The cytokine profile in active UC shows significant interferon-γ release but is not as polarized as that seen in CD. It is currently accepted that UC is an untypical Th2-dominated disease. The proinflammatory cytokines IL-6, IL-8, IL-10 and tumor necrosis factor are increased in both active UC and CD.

Nature’s most powerful probiotic:
Helminth Parasites and Regulatory Mechanisms Of Immune Response
Parasitic helminths represent an extreme in the spectrum of pathogens, as large multicellular animals derived from free-living metazoan ancestors. Although commonly grouped together, the helminths in fact comprise two very distantly related taxa that diverged 600 million or more years ago, ie, the roundworm nematodes and the flatworm plathelminths. Between these two main groups of distantly related helminth parasites, individual species of parasites have evolved to occupy a diverse range of niches within their hosts, using a wide range of infection strategies, yet with few exceptions the mammalian host responds to these diverse groups of organisms in a remarkably consistent and even stereotypical manner. Typically, this response involves the production of the cytokines interleukin-4 (IL-4), IL-5, IL-10, and IL-13, as well as immunoglobulin E (IgE) and the expansion and mobilization of specific effector cells, such as mast cells, eosinophils, and basophils. Collectively, this group of responses resembles the T-helper 2 (Th2) immune response. Th2 responses may serve the host by limiting the degree of helminthic organization.
It is speculated that helminths have developed these methods of manipulating the immune response to evade immune-mediated destruction and thus to survive. However, it is unlikely that helminths simply alter the Th1/Th2 balance. Recent studies indicate that helminths stimulate the development of regulatory T-helper cells (Treg) that reduce both Th1 and Th2 responsiveness. Treg regulate immunity through both cytokine-dependent and independent mechanisms. By stimulating the secretion of IL-10 and transforming growth factor-13 (TGF-P) they suppress the differentiation of both, Th1 and Th2 lymphocytes. The immunoregulatory cytokines are involved in controlling intestinal inflammation although the details which cells produce these cytokines under what condition are not yet fully understood.
IL-10 inhibits the function of both macrophages and dendritic cells, including their production of proinflammatory cytokines and thus has potent anti-inflammatory properties. Defects of these regulatory mechanisms lead to the development of specific Th1- or Th2-mediated diseases.
Helminthic Therapy:
Hygiene Hypothesis and IBD
The history of helminths living in the intestinal tract or other locations of their host can be traced back to the earliest record of human beings, and colonization of humans with these organisms was nearly universal until the early 20th century. Although effective preventive and therapeutic measures have been developed for most parasitic worms, helminth infections are still very common in the developing world today. It has been estimated that more than one billion individuals worldwide are infected with one or more helminths, with the highest frequency found in children subjected to poor sanitation. The hygiene hypothesis postulates that multiple childhood exposures to enteric pathogens protect an individual from autoimmune diseases (e.g. IBD) later in life, whereas individuals raised in a more sanitary environment are more likely to develop immunological disease like IBD. Modern
hygienic practices and absence of exposure to intestinal helminths appears to be an important factor contributing to immune dysregulation and increased susceptibility to immunological diseases. This theory was originally expressed as the “Old friends”-hypothesis.
Epidemiological studies show that like other autoimmune diseases, IBD is most common in Western industrialized countries that are characterized by affluent societies living in sanitized environments. IBD is rare in developing countries with less clean environments and more crowded living conditions. These epidemiologic findings suggest that the prevalence of IBD is inversely correlated to the prevalence of helminthic parasites in that population.
Helminth parasites are the classic inducer of Th2 responses. The Th2-polarized T cell response driven by helminth infection has been linked to attenuation of damaging Th1 driven inflammatory responses. This may prevent some Th1-mediated autoimmune diseases with overly active Th1 inflammatory response such as occurs in CD. In addition to stimulating vigorous Th2 response, helminth infections are also capable of inducing suppressive T-cell populations known as regulatory T cells (Treg), which may help control morbidity and dampen resistance to reinfection through their potent immune regulatory mechanisms. Genetically predisposed persons who never were exposed to helminths might lack a strong counteractive Th2 immune response and Treg cell clones, rendering the intestinal immune system more prone to Th1-mediated diseases like CD.

Nature’s most powerful probiotic:
TSO® in the Treatment of IBD
Under experimental conditions, a number of helminths have been shown to induce Th2-type cytokine release and also to downregulate the Th1 immune responses to unrelated bacterial and viral infections. Epidemiologic evidence, case control observations, animal studies and clinical studies all suggest that helminths may afford protection from or even treat intestinal inflammation. Therefore, colonization with intestinal helminths might be beneficial in reducing inflammation in patients with IBD. An ideal biological agent should colonize the intestine without invading the host and should have little or no pathogenic potential. The source of the organism should be pathogen-free to minimize the potential of co-transmitting other diseases and it should not be a public health hazard to cohabitants or other close associates.
According to experiments performed thus far the porcine whipworm Trichuris suis seems to be the most attractive candidate for development in IBD. T. suis is genetically related to Trichuris trichiura, the human whipworm, but it is not a human pathogen. In humans T. suis has no systemic phase, does not multiply in humans, is not directly transmittable by contact, and is cleared spontaneously. It has been shown experimentally that T. suis is able to colonize humans briefly without causing disease symptoms.
Helminthic Therapy:
Physical and Chemical Properties
TSO® is a natural probiotic product. It is presented as a non-sterile aqueous suspension of the viable embryonated eggs of the whipworm Trichuris suis for oral use. The eggs are suspended in an isotonic phosphate buffered saline solution of pH2.4. TSO® bottles contain either 500, 1000 or 2500 viable Trichuris suis ova (TSO®) in 15 ml of the suspension supplied in a 30 ml glass container with polypropylene screw cap.

Nature’s most powerful probiotic:
Storage Conditions
TSO® has actually 2 kind of shelf life. One is the biological shelf life which is the time that they stay viable and one is the microbiological shelf life of the fluid the TSO® organisms are floating in. In their natural life cycle the eggs are shed by their natural hosts (the pigs) into the soil where they can stay good for up to 9 years while exposed to all kind of weather conditions from very hot to very cold.
When the next pig picks them up they attach to the mucosa, hatch and start their development. The microbiological shelf life refers to the bacteria load of the fluid inside the bottles until they exceed the limits according to the pharmacopeia for fluid medicines, which is at least 1 year depending on the condition the vials are stored. If they stored inside a fridge at between 4°C/39°F and 8°C/47°F this time can be a lot longer.
Helminthic Therapy:
Nonclinical Studies
Initial nonclinical studies with TSO® were aimed to identify appropriate and relevant animal models mimicking the transient colonization of T. suis observed in humans. The monkey and the rabbit were identified as relevant animal models and were used in the main program of pharmacological and toxicological studies. Due to the lack of disease models in these species all studies with TSO® were conducted in healthy animals. However, studies with other helminths mainly conducted in rodent models provide insight in several immune-modulatory mechanisms that are probably responsible for the therapeutic effect.

Nature’s most powerful probiotic:
Nonclinical Pharmacology
Influence of helminthic infection on immune-mediated disease
Animal models of human disease provide insight into the mechanisms that drive illness and help identify therapeutic interventions. Animal models of immune-mediated disease share certain features:
– immune dysregulation produces excessive inflammation,
– excessive inflammation results in organ dysfunction, and
– suppressing immune cell function or restoring immune regulatory pathways reduces inflammation and organ damage.
The effect of helminth exposure has been tested in animal models of IBD and other diseases like asthma, multiple sclerosis and autoimmune (type 1) diabetes. The initial work by Elliot, et. al., showed a protective response of Schistosoma mansoni infection on trinitrobenzene sulfonic acid (TNBS)-induced colitis in mice, a chemically induced Th1 -type colitis used as an experimental model of human IBD, have led to several animal studies determining the role of helminth infection in different IBD models. Following observations with different nematode, cestode and trematode provide evidence to suggest that helminth parasites can ameliorate chemically induced colitis in different models. Protection from colitis in these models requires intact host IL-4 circuitry and IL-10 signaling.
The rodent models suggest that protective immunity against gastrointestinal nematodes requires CD4+ T cells that release factors that orchestrate protective host pathways. Helminths induce Th2-mediated immune responses, which are characterized by the production of high quantities of IL-4, IL-5, IL-10, and IL-13, and consequently induce the development of strong IgE, eosinophilic, and mast cell responses.
Humphreys, et.al., recently identified IL-33 as a potential link between innate and adapted immunity to intestinal parasite infection. They showed in mice that IL-33 is produced very early during Trichuris muris infection and plays two independent roles in the context of nematode infection. IL-33 provides the initial impetus for Th2 polarization and acts as a T cell-independent pro-inflammatory cytokine at the site of infection, influencing the gut tissue inflammatory response to parasite invasion.
Moreover, helminths also induce the development of immunomodulatory Treg cells secreting IL- 10 and transforming growth factor-1 (TGF-1).
Helminthic Therapy:
Immunological host response to Trichuris suis
The host response to Trichuris suis was studied in the pig which is the natural host of T. suis as well as in the monkey and the rabbit, which are foreign hosts but allow a transient colonization with T. suis. Because of the transit nature the monkeys and rabbits are considered as appropriate models for the treatment of humans with TSO®.
As expected for a parasitic colonization, a marked increase in blood eosinophilic granulocytes was noted in all three species. Levels of eosinophilic granulocytes peaked 6-8 weeks after first administration in pigs and within 3-4 weeks after first administration in monkeys and rabbits probably reflecting the earlier expulsion from the foreign hosts (monkey and rabbit). White blood cell counts and levels of lymphocytes, neutrophils and monocytes remained unaffected. Only in rabbits a transient decrease of basophilic granulocytes was noted in parallel to the increased eosinophilic granulocytes. In monkeys treated every two weeks with 500 or 2500
TSO®/kg the increase of blood eosinophilic granulocytes was dose-dependent.
In pigs and rabbits, a marked infiltration of eosinophilic granulocytes was also observed in the lamina propria and the submucosa of the caecum and anterior part of the colon, which are the target sites of T. suis. The adjacent regions of ileum and distal colon were affected to a lesser extent. In addition, an increased infiltration of mast cells was noted in the proximal colon of pigs colonized with T. suis. Only slight effects were noted in monkeys, probably due to the relatively high spontaneous infiltration rates of eosinophilic granulocytes and mast cells in the intestinal mucosa of the animals. Formation of antibodies directed against the excretory/secretory (E/S) antigen of T. suis was observed in all species. In pigs, levels of E/S-Antigen specific IgG1, IgG2 and IgM peaked approximately 9 weeks after administration of a single dose and declined after worm expulsion, while IgA rose earlier and remained elevated after expulsion. In monkeys E/S antigen specific IgG peaked 6 weeks after beginning treatment with either 500 or 2500 TSO®/kg administered every two weeks. In rabbits, peak levels of E/S antigen specific IgG were reached within 4 weeks after treatment with 2500 TSO®/kg every two weeks. Mean antibody titers appeared to be correlated to the TSO® dose.
In all three species the treatment with TSO® led to induction of a Th2 immune response. In pigs the stimulation of peripheral blood mononuclear cells (PBMC) with T. suis E/S antigen led to a pronounced increase in IL-4 secreting cells from week 4 to week 8 after administration. Secretion of IL-10, IFN-γ and TNF-α from E/S antigen activated PBMC was not affected. In rabbits, an increased expression of IL-4 and IL-13 in PBMC was demonstrated approximately 4 weeks after start of TSO® treatment, while expression of IL-10, interferon gamma (IFN-γ) and TGF-f3 remained unaffected. Monkeys demonstrated a pronounced Th2 response already 1-2 weeks after the first administration of TSO®. In PBMC, activated in vitro with T. suis E/S antigen, a marked increase of secretion of IL-4, IL-5 and IL-13 was noted. Secretion of IL-2 and IFN-γ from activated PBMC was elevated to a less extent. No difference to the control group was observed for secretion of IL-10, IL-12, IL-17, IFN-γ, TGF-P, and TNF-α from activated PBMC. The increase in cytokine secretion from E/S antigen activated PBMC appeared to be dose dependent. The secretion of Th2 cytokines from activated PBMC remained elevated through the dosing period.
An increased expression of Th2 cytokines was also observed in the intestinal mucosa at the site of colonization. In pigs, elevated levels of IL-4, IL-5 and IL-13, but not IL-10 mRNA were observed in the mucosa of the proximal colon 2-11 weeks after administration of a single TSO® dose. In vitro treatment of intestinal pig epithelial cells (IPEC-1) led to production of IL-6 and IL-10 within 24 hours. In rabbits increased IL-13 mRNA levels were detected in the mucosa of the proximal colon at week 7 after repeated treatment with TSO®. Levels of IL-4, IL-10, IFN-y and TGF-13 mRNA were not changed.
Treatment with TSO® did not change the pattern of lymphocyte types in peripheral blood of pigs, rabbits or monkeys at various stages of the TSO® treatment.
These studies in pigs have demonstrated that the immunological host response is due to treatment with viable embryonated TSO®. None of the above described effects was seen after treatment with inactivated TSO®.


Nature’s most powerful probiotic:
Safety Pharmacology
As has been demonstrated in pigs, monkeys and rabbits and as it is expected for humans, the colonization with T. suis is confined to the mucosa of the caecum and colon. An aberrant migration, which would result in systemic exposure, was neither observed in pigs nor in monkeys and rabbits.
The effect on cardiovascular and circulatory function was studied in all animals of the
subchronic toxicity study in cynomolgus monkeys at various time-points prior to and after the dosing as well as at the end of the recovery period. No effect of the treatment with TSO® on the cardiovascular and circulatory function was noted.
Helminthic Therapy:
Pharmacokinetics and Drug Metabolism in Animals
There is no pharmacokinetic information regarding TSO®, as neither the ova nor the developing larvae and mature worms are systemically absorbed, and thus measurement of concentrations in blood is not relevant. In lieu of pharmacokinetic data, the life cycle of the whipworm is provided as follows:
Following ingestion of infective T. suis ova by pigs (natural host), all subsequent larval development occurred in the mucosa of the caecum and colon. Eggs hatched in the distal region of the small intestine and throughout the large intestine. Twenty-four hours after ingestion the majority of the infective eggs were found in the large intestine, larvae were seen in the intestinal contents. Three days after infection 85% of eggs remaining in the colon had hatched but no larvae were found in the intestinal contents after the 5th day. Larvae penetrated the colonic mucosa via the crypts of Lieberkühn, where they entered the cells lining the crypts. The growing larvae entered the lamina propria and became equally distributed between crypt cells and cells of the lamina propria.
Approximately 10 days after hatching the larvae migrated to the mucosal surface. Damage to the lamina propria appeared to be very localized and slight as the larvae only appeared to disrupt the crypt and lamina propria cells in which they were lying. No evidence of any visceral migration could be found. From the 28th day, the larvae were found lying in a shallow depression on the mucosal surface covered by a raised sheath of mucosal epithelial cells. Fully formed eggs were first observed in the uterus of female worms on day 41; this was the earliest date that eggs were detected in the feces of the host. Little or no host tissue reaction was seen in any of these infections. Expulsion of the mature worms from the host occurred between week 9 and 11 after ingestion. In monkeys and rabbits, which are considered as appropriate models for TSO® treatment in humans, the majority of the ingested TSO® hatched after ingestion. Hatching occurred mainly in the cecum and the anterior part of the colon. Twelve hours after ingestion the larvae were associated with the mucosa of the caecum and the anterior part of the colon. Also at later stages, similar to the pig, the colonization of the gut mucosa was confined to the caecum and colon; a colonization of the upper part of the gastrointestinal tract was not observed. Detailed macroscopy and histopathology of other organs gave no evidence for an aberrant migration of T. suis into visceral tissues in monkeys and rabbits. After administration of a large single TSO® dose, larvae remained established in the mucosa of the large intestines for at least 2 weeks in the rabbit. In the monkey the time-point of expulsion was variable: several animals expelled T. suis larvae within 10-15 days, while remained established in other animals over 20 days. Larvae were not detected in the intestines at later time-points after ingestion of a single dose indicating only transient colonization and expulsion of before reaching the adult larval stages. The early expulsion from the foreign host is also reflected by an earlier onset of the immunological host response seen in monkeys and rabbits compared to pigs.
Mature worms or an excretion of eggs in the feces of the hosts, which would have indicated sexual maturity of the larvae, were neither observed in monkeys nor in rabbits. After multiple dosing (7 doses) in monkeys every other week simulating the schedule in clinical trials, larvae were not detectable in the intestinal mucosa one week after the last dosing, which indicates that larvae are digested and expelled earlier after induction of the host immune response.

Nature’s most powerful probiotic:
Toxicology
Pathology of porcine Trichuriasis
Broad experience from production and potency testing of TSO® in mini-pigs and studies on experimental colonization of mini-pigs and normal pigs demonstrated that the treatment with doses of TSO® (range: 2000-2500 TSO/animal; 125-380 TSO®/kg) was well tolerated. Negligible clinical signs in form of softened feces were occasionally observed in these studies.
The host response was characterized by increased eosinophilic granulocytes, slight increase in neutrophils and monocytes, formation of T. suis specific antibodies and increased levels of 13- globulin and γ-globulin. At necropsies the pigs showed high numbers of T. suis larvae/worms associated with the intestinal mucosa of the caecum and colon suggesting that the majority of the administered TSO® dose was able to colonize. Apart from a slightly edematous, reddened and thickened mucosa observed in the infested regions in some of the TSO® treated animals no macroscopic changes were observed. As seen at histopathological levels, the mild hypertrophy of the intestinal mucosa was accompanied by increased crypt lengths, increased infiltration of mononuclear cells (lymphocytes, histiocytes and plasma cells) an increased activation of lymphatic tissue as well as a marked infiltration of eosinophilic granulocytes in the lamina propria and tela submucosa. The above described changes were partly or completely reversed within 10 days after an anthelminthic treatment of the colonized pigs with mebendazole. In contrast to the subclinical symptoms observed in pig trichuriasis, severe symptoms are described for heavy natural infections in young pigs and after experimental colonization of pigs (age: 7-10 weeks) with very high doses of TSO® (25,000 – 400,000 TSO®/animal).
Clinical symptoms observed in these severe infections include anorexia, growth retardation, weight loss, bloody and mucoid diarrhea, dysentery, rectal prolapse, anemia, emaciation and death. Affected pigs had severe mucohemorrhagic catarrhal enteritis with pseudonecrotic membranes in caecum and colon. Apart from the TSO® dose and the age of the pigs an important determinant for the presence of severe clinical signs appears to be whether or not a secondary microbial invasion occurs.
Helminthic Therapy:
Single and Repeated Dose Studies
The pig was generally not considered as a suitable animal model for toxicity studies with TSO® as pigs are the natural hosts of T. suis allowing a highly efficient permanent colonization and a full maturation to adult worms. Non-natural hosts as monkeys and rabbits which are susceptible to a transient colonization with T. suis similar to the transient colonization in humans have been identified as relevant models for toxicity studies. Based on the pharmacodynamic and pharmacokinetic characteristics and the close similarity with the human regarding anatomy, physiology and immune function, the monkey is considered as primary toxicity model for TSO®. The rabbit was selected as an additional model for reproduction toxicity studies.
Several exploratory studies employing various TSO® doses and treatment schedules were conducted in monkeys and rabbits (up to 25,000 TSO®/kg in monkeys and up to 33,000 TSO®/kg in rabbits). In all studies TSO® was well tolerated and did not provoke signs of systemic toxicity. The clearest sign of the host reaction to T. suis was a marked increase of eosinophilic granulocytes in the peripheral blood (peaking around day 20 in the monkey and day 30 in the rabbit) as well as a marked infiltration of eosinophilic granulocytes into the mucosa and submucosa of the caecum and colon which are the target regions of T. suis. In some of the treated animals, small focal alterations were observed in the otherwise unaffected mucosa of the caecum and colon. The occurrence of the focal alterations did not show a clear dependence on the dose or dosing frequency. The focal alterations presented as small red discolored foci (size usually 1-2 mm) and were in some cases partly thickened and indurated.
The foci were considered to be probably related to T. suis; however, at least in monkeys similar alterations occurred also spontaneously in control animals. As no clear histopathological changes were observed, in particular no epithelial damage, the focal alterations were considered to be of no pathological relevance. A general inflammation of larger parts of the intestinal mucosa was not observed even when the animals were treated with high TSO® doses of 25,000 TSO®/kg (monkeys) or 33,000 TSO®/kg (rabbits). In rabbits, increased mesenterial lymph nodes and after treatment with a high dose also thickened Peyers patches were observed.
A 13-week subchronic toxicity study with TSO® was conducted in cynomolgus monkeys. Six animals per sex/group were orally treated every two weeks with placebo, 500 TSO®/kg or 2500 TSO®/kg. According to the mean body weight, the female animals received in total 7 doses of 1500 TSO® or 7500 TSO®, while the male animals received 7 doses of 2250 TSO® or 11500 TSO®, respectively. Scaled on a dose per body weight base the highest dose in the subchronic toxicity study was more than 20-fold above the highest dose proposed in future clinical studies (7500 TSO® or 107 TSO®/kg). Four animals per sex/group were sacrificed after 13 weeks, while the remaining animals were sacrificed after a 12-week recovery period.
During treatment the typical host response to TSO® was noted in a dose-dependent manner. However, no signs of treatment related toxicity was noted. In particular, treatment with TSO® did not influence clinical signs, mortality, body weight, food consumption, body temperature, hematological parameters (except eosinophilia), blood coagulation, urinary parameters, clinical biochemistry parameters, ophthalmological and auditory parameters and organ weights. TSO® did not affect the level of total IgE. No diarrhea or increased frequency of occult blood in stool was noted in the treatment groups. Only in the male animals of the high dose group on day 40 of treatment showed a tendency to softened feces was noted, however the fecal consistency was considered to be in the normal range. At macroscopy, 8 days after the last treatment, no larvae were detected in the intestinal mucosa indicating early expulsion of the larvae after repeated dosing. In a subgroup with 4 animals treated with a single TSO® dose, a high hatching rate and colonization of the caecal mucosa with T. suis was demonstrated. In animals of all groups small focal alterations, as described above, were observed in the otherwise unaffected mucosa of the caecum and colon.
However, the frequency was higher only for the females of the low-dose group when compared to the control group. A detailed macroscopical and microscopical inspection of other organs did not reveal any evidence for an aberrant migration of T. suis into other tissues. Two of the female animals of the high dose group showed increased mesenteric lymph node weights, which subsided in the recovery period. At the histopathological level a slight trend towards increased infiltration of eosinophilic granulocytes and mast cells into the mucosa of caecum and colon was noted for the male monkeys. An increased infiltration of eosinophilic granulocytes was also noted in mesenteric lymph nodes of single TSO® treated animals. No further histopathological changes were noted. The observed changes in the treated monkeys were considered to be the expected (pharmacodynamic) host response. None of the findings was considered to be of pathological/toxicological relevance. After repeated treatment of monkeys with TSO® every other week over 13 weeks a NOAEL (no-observed adverse effect level) of > 2500 TSO®/kg can be concluded (> 7500 TSO®/female, > 11250 TSO®/male animal).


Nature’s most powerful probiotic:
Reproductive and Development Toxicity
A study in rabbits was conducted to obtain information on the influence of the treatment with TSO® on the fertility and embryo-fetal development by oral administration to the animals of the Fo generation (24 animals per group and gender). TSO® was administered every second week during the pre-mating and the mating periods at dose levels of 500, 2500 and 7500 TSO®/animal corresponding to 167, 833, and 2500 TSO®/kg. During gestation the female animals were dosed weekly to detect any acute/short-term effects of the treatment on the critical phase of
organogenesis. The animals demonstrated the typical host response to T. suis demonstrating an increase in peripheral eosinophilic granulocytes, a transient decrease of basophilic granulocytes, a marked infiltration of eosinophilic granulocytes in the mucosa of the caecum and colon and into mesenterial lymph nodes, as well as a dose-dependent occurrence of T. suis specific IgG.
At macroscopy after 8-9 weeks, a dose-dependent colonization of the caecum mucosa with T. suis larvae was observed. No focal alterations were noted in the intestines and a detailed macroscopical and histopathological investigation of other organs did not reveal evidence for an aberrant migration of T. suis. No test-item related influence was noted on mortality, clinical signs, body weight or food consumption. Apart from a slight, non-significant increase of mesenteric lymph node weights in the female animals of the high dose group no test-item related changes in organ weights were noted. Treatment with T. suis had no influence on stool consistency or the occurrence of occult blood in stool.
No treatment related influence was noted on mating behavior, fertility, sperm parameters, implantation or embryo-fetal development. No teratogenic properties were noted up to the highest dose. There was no treatment related increase in the incidence of fetal malformations, external / internal, skeletal or soft tissue variations or skeletal retardations. In conclusion, treatment with TSO® induced the expected host reactions, but no adverse effects in the parent generation. Treatment with TSO® had no effect on fertility and embryo-fetal development and did not express teratogenic properties. The NOAEL on the parent generation and the fetuses was > 2500 TSO®/kg (> 7500 TSO®/animal). In addition to the preclinical study, fertility parameters were tested in the subchronic toxicity study in monkeys. No TSO®-related effect on the estrus cycle was noted and the sperm analysis did not reveal test-item related changes. No treatment-related histopathological changes were observed in the reproductive organs of TSO®-treated male and female monkeys.
Helminthic Therapy:
Special Studies
Since the broad-spectrum anthelminthic mebendazole is the recommended drug for treatment of human Trichiasis its effectiveness against T. suis was studied as an antidote. The study was conducted in Goettingen mini-pigs as these animals allow a permanent colonization with high numbers of T. suis larvae/worms. Monkeys and rabbits were not considered appropriate for this study as these non-natural hosts allow only a transient colonization with T. suis with early and spontaneous digestion and expulsion. Two groups of minipigs (4 male and 4 females/group) were treated with a single dose of 2500 TSO® (300-380 TSO®/kg).
Fifty days after administration of TSO® one group was treated with 2×100 mg mebendazole/day over 4 days, which is the recommended treatment for human trichuriasis (according to the prescribing directions for Vermox®) while the other group served as control. Treatment with mebendazole led to a complete elimination of T. suis in all animals of the group as was demonstrated by a 100% reduction of fecal egg excretion and complete absence of intestinal T. suis larvae/worms at dissection.


Nature’s most powerful probiotic:
Other Toxicity Studies
The fact that TSO® and resulting T. suis larvae are confined to the intestine rules out a systemic exposure. TSO® did not appear to be an irritant. TSO® treatment is contraindicated in subjects known to be hypersensitive to Trichuris species or compounds made of Trichuris species.
There are no studies on cross-reactivity between T. suis antigens and allergens. Potential crossreactivity is not likely to have an effect on allergen-specific IgE levels, since T. suis is confined to the intestinal tract, where IgA responses dominate. In the subchronic toxicity study in monkeys no effect on total IgE was noted.
Helminthic Therapy:
Summary of Toxicity Studies
Monkeys and rabbits were identified as relevant animal models for TSO®. Both species demonstrated a transient colonization with T. suis, which was confined to the mucosa of the caecum and colon. No aberrant migration into other tissues and no signs of systemic toxicity were observed. The typical host reaction was characterized by an increase of eosinophilic granulocytes in peripheral blood and infiltration of eosinophilic granulocytes in the mucosa of caecum and colon, the formation of T. suis antigen specific IgG, and an increase in expression of Th2 cytokines in activated peripheral blood mononuclear cells and intestinal tissue. In some treated animals minor focal alterations in the otherwise unaffected mucosa of the caecum and colon were observed. As no clear histopathological changes were observed, in particular no epithelial damage, the focal alterations were considered to be of no pathological relevance. However, as the mucosa was otherwise unaffected and as no general inflammation of larger parts of the mucosa were observed, the small focal alterations were not considered as local intolerance reactions.
Neither diarrhea nor an increased frequency of occult blood in stool was observed. No systemic or local adverse effects were noted up to a dose of 2500 TSO®/kg when administered every two weeks over 13 weeks. Treatment with TSO® had no effect on fertility and embryo-fetal development and did not express teratogenic properties at a dose of 2500 TSO®/mg, given every two weeks. In conclusion, the nonclinical studies with TSO® in monkeys and rabbits did not cause a safety concern for the planned clinical trial. It was demonstrated in pigs that mebendazole when used in the dose regime recommended for human Trichiasis is also effective against T. suis. Therefore, if elimination of T. suis from patients is necessary, mebendazole appears to be the antidote of choice.

Nature’s most powerful probiotic:
Effects in Humans
Originally, the rationale for performing research with helminths was based on the hygiene hypothesis, which was refined from the “Old friends Hypothesis” for diseases resulting from disorders of the immune system, which postulates that multiple childhood exposures to parasites and pathogens protect an individual from allergic and autoimmune disease later in life, whereas individuals raised in a more sanitary environment are more likely to develop autoimmune diseases and allergies. In line with these hypotheses, epidemiologic evidence, case control observations, animal studies and clinical studies all suggest that exposure to helminth parasites which followed the whole human evolution since its early beginnings may afford protection from or even treat autoimmune disorders and also IBD. For example, Radon e.t al., 2007 conducted a case-control study to test the association between farm animal contact in infancy and development of juvenile CD and UC.
The results of 444 case subjects with CD, 304 case subjects with UC and 1481 control subjects confirmed that regular contact with farm animals during the first year of life was associated inversely with CD and UC. Infections with the human parasites are rare in developed countries; when infections are found in developed countries, they are almost exclusively found in travelers or in immigrants. Safety and efficacy of TSO® have been investigated in clinical studies with patients suffering from IBD, Multiple Sclerosis, allergies and other autoimmune diseases. The results of these previous trials indicate that Trichuris suis therapy might be effective in both forms of IBD, CD and UC. Results of treatment with a single dose were temporary in some patients, but it was possible to maintain a remission with a scheduled administration of TSO® every 2-3 weeks for longer periods.
Crohn’s disease is a heterogeneous chronic disorder of the bowel characterized by relapsing inflammatory process. In contrast to ulcerative colitis, inflammation may be patchy and segmental, and typically transmura1. Mucosal inflammation generates large amounts of IFNγ and TNFα suggesting that excess production of Th1-type cytokines is one common mechanism underlying the pathogenesis of disease. Immune response to helminths promotes Th2-response and exposure to TSO® may prevent an exuberant Th1 inflammation at mucosal surfaces like that seen in CD.
Ulcerative colitis is a relapsing non-transmural inflammatory disease restricted to the colon and rectum. Dependent on the anatomic extent of involvement, patients can be classified as having proctitis, procto-sigmoiditis, left-sided colitis (involving the sigmoid colon with or without involvement of the descending colon), or more extended colitis. The pathophysiology of this disorder is multifactorial and incompletely understood. It seems that the disease results from inappropriate activation of the mucosal immune system, resulting in the inflammatory responses.
In contrast to CD, UC is considered an atypical Th2 more than a Th1 response. The former idea of helminthic action was that hosts develop a Th2 response, which blunts over-reactive Th1 pathways. Recent data from animal studies suggest that helminths impede Th1 and Th2 responses generating an immunoregulatory environment via Treg cells, (mediated via IL-10 and/or TGF-β. Differences in the mucosal infiltration by Treg cells of Helminthiasis and subsequent active UC were found by immunohistochemistry in a 12-year old girl. Treg cells were found to be increased in UC compared to normal mucosa. Development of inflammation after worm clearance is accompanied by a clear reduction of mucosal Treg cells.
Helminthic Therapy:
Pharmacokinetics and Drug Metabolism in Humans
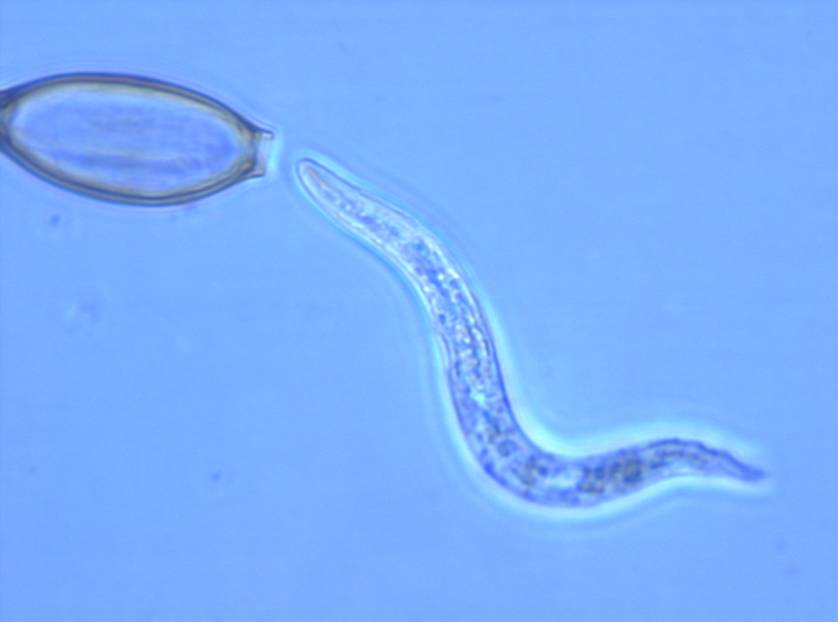
Fate of TSO®/ T. suis in humans
The microenvironment of the intestine induces the hatching of larvae from embryonated eggs and the release of the first-stage juvenile larvae of Trichuris suis. The eggs have two polar plugs, which are detached/opened upon hatching. Preparations of TSO® for clinical application consist of more than 80% embryonated eggs. Non-embryonated eggs will by nature not be triggered to hatch. The manufacturing process controls embryonation which occurs after >5-6 weeks. Under natural conditions, the eggs embryonate in soil after >5-6 weeks, provided conditions are favorable. Only after embryonation the eggs are able to hatch in humans. To the best of our knowledge T. suis eggs are not absorbed systemically and Trichuris suis has not been shown to be invasive.
By nature, neither the eggs nor the larvae are converted chemically at the intestinal wall or other digestive processes. Dead larvae are digested like food proteins. Therefore, the traditional model of pharmacokinetics (absorption, plasma protein binding, distribution, elimination, and bioavailability) is not applicable. Egg excretion has not been detected in regular stool samples from IBD patients but has been reported by Beer. No living worms or larvae have been found in stool samples or during endoscopy in studies done by Summers, et. al., in IBD patients. There is a single case report about detection of an adult Trichuris by biopsy in a 16-year old patient who underwent TSO® treatment for CD. Segments of immature larvae were identified directly beneath attenuated ileocecal mucosal epithelium. The site of colonization and the ultrastructural localization within the epithelial surface are similar to the migration of T. suis in pigs. Since the worm was identified free-floating in the lumen, without evidence of mucosal attachment, it may have been in the midst of being expelled from the bowel.
Up to now to the best of our knowledge no cases of symptomatic or pathologic invasion or infestation of T. suis were described neither in humans nor in other non-natural hosts like rabbits or monkeys. Based on the experience with T. suis in humans, the possibility of errant larvae of T. suis establishing in vital organs or sites in humans seems unlikely.

Nature’s most powerful probiotic:
Drug Interactions
The potential of interactions with drugs is unknown/unlikely. We are not aware of any literature report on drug interactions with Trichuris suis. In clinical trials T. suis was used in patients who concomitantly used salicylates, steroid or thiopurines without evidence of worrisome interactions.
Helminthic Therapy:
Overdose
Clinical signs and symptoms of overdose have never been reported. A description of overdose is unknown. Since the broad-spectrum anthelminthic mebendazole is the recommended drug for treatment of human Trichuriasis and since mebendazole has been demonstrated to effectively eliminate T. suis from pigs when administrated at the dose regime recommended for human patients, mebendazole is considered to be the antidote of choice.


Nature’s most powerful probiotic:
Effect of Concomitant Food Intake on TSO®
As TSO® is not systemically absorbed, and thus there are no considerations for the effect of food on luminal dissolution, drug permeability and systemic availability of TSO®. Transit time of the eggs through the GI tract could be impacted by food; however, as the rate of transit is not necessarily important (given the eggs are intended to remain in the gut for weeks at a time), a delay in transit due to food intake is not considered a material issue for TSO® treatment. Therefore, treatment with TSO® can be given without regard to concomitant food intake.
Helminthic Therapy:
Effect on QT Interval
TSO® is not systemically absorbed and therefore is not expected to have systemic bioavailability. The distribution of TSO® is relegated to the gut, and specifically the intestinal tract. In light of this, TSO® is not expected to affect QT interval.


Nature’s most powerful probiotic:
Safety and Efficacy
No clinically significant changes in the blood count or hepatic tests occurred in any subject at any time during the three clinical studies with TSO® in IBD patients. Notably, half of the patients treated in these studies were reported to have been receiving concomitant corticosteroids, azathioprine/6-mercaptopurine, or both. In the allergic rhinitis study (Bager, et. al.), treatment-emergent adverse events occurred in 83 (86%) of the 96 subjects that were eligible for analyses, and the most frequent events were gastrointestinal disorders (228 of 514 events, 44%). Significantly more subjects in the TSO® group experienced a non-serious gastrointestinal disorder (76% versus 49% for placebo, p=0.007). The most frequent type experienced was diarrhea (47% versus 32% for placebo, p=0.13), while upper abdominal pain (37% versus 4% for placebo, p<0.001) and flatulence (43% versus 17% for placebo, p=0.005) were significantly more frequent in the TSO® group.
No subjects were hospitalized due to gastrointestinal disorders caused by TSO®. Contrary to the closely related human parasite Trichuris trichiura, T. suis appears not to propagate in the human intestinal tract. Based on the experience with T. suis in humans possibility of aberrant larvae of T. suis establishing in vital organs or sites of humans appears unlikely. If symptoms should occur, the causative agent could be easily treated with antihelminthics.
Helminthic Therapy:
Literature Case Reports and Reviews
Early data on the tolerability of TSO® in humans was obtained after an experimental self-infection of a 23-year old healthy individual with TSO®. Beer (1971) reported in the British Medical Journal a case of experimental self-infection of T. suis in a 23 year old male. A single oral dose of 1000 eggs were taken orally. The patient experienced no symptoms of distress and no diarrhea. There was no change in the differential white count. Beer reports an infection was established by day 60 with recovery of approximately 20 eggs per gram of stool following the estimated time for maturation. Beer reported that humans were able to be infected with T. suis. This observation was one of several considerations that led to the original proposal that T. suis would be a good therapeutic choice.
Kradin et al (Kradin 2006) found what he and his colleagues described as a sexually mature adult trichurid by cecal biopsy in a 16 year old patient who underwent TSO® treatment for CD. They identified immature helminths in the ileocecal region. However, they concluded that the presence of an adult male helminth in the cecum indicates that maturation to the sexual stage of T. suis does occur in man. Summers et al (2006) commented on this case by stating it was highly unlikely that T. suis causes prolonged colonization of humans. In their studies of T. suis ova therapy in ulcerative colitis and Crohn’s disease, they performed colonoscopy in some patients and occasionally saw helminths of variable size and maturity. Ova in the stool were never detected despite regular examinations, suggesting that the worms never reached maturity. In patients who had been off ova therapy for several months, including some patients who were taking various immunosuppressants, they never saw eggs in the stool or parasitic forms on colonoscopy. Also, even if eggs are produced, they will be immature. They cannot autoinfect the host or directly transmit infection to people nearby; the eggs need to rest in moist soil for perhaps a month to reach maturity and become the infective form. Normal hygienic practice will prevent any transmission of infection because only immature eggs can be passed in the stool. Once again, even farmers who raise pigs do not get sick from T. suis exposure. Farmers who are frequently exposed to T. suis by close contact with colonized pigs have never develop clinically associated disease or chronic T. suis colonization. Thus, Kradin suggestion that prolonged parasitic colonization may be a concern is speculation.
Van Kruiningen and West (Van Kruiningen 2005) speculated that aberrant migration of the developing larvae could occur. However, based on the biology and structure of the T. suis larvae, Reddy & Fried (2009) consider it unlikely that T. suis could enter the human lymphatic or venous system by means of the gut submucosa. There are no observations of T. suis producing disease in humans. Humans have cohabitated with pigs for centuries. In unhygienic areas where porcine stool is distributed onto the soil, eggs from these infected pigs can reach maturity in the surrounding soil. People would have been constantly exposed to viable mature eggs. A complete lack of reported disease suggests they are remarkably safe. In addition, Trichuris trichiura has not been reported to migrate aberrantly even in HIV-infected individuals in Africa.
Van Kruiningen cites Henry (Henry 1970) as an example of aberrant migration in an unnatural host. “When T. suis was identified in wild boars (Sus scrofa), a foreign host for them, they had traveled an aberrant pathway to as far as the kidneys”. However, the Appalachian wild boar is the same animal as the domesticated pig; the American wild boar is simply a feral pig (Susscrofa). Thus, it is not a “foreign host”. Furthermore, the Henry report is from field autopsies of killed trapped boars. The helminth they found on top of a kidney likely resulted from artifactual spillage rather than true migration. Aberrant migration has not been reported in domestic pigs
(same host).
Hsu et al (Hsu 2005) raised the theoretical concerns regarding the fate of TSO® in patients who do not respond to therapy (theoretical risk:benefit ratio). Summers et al (Summers 2006) countered this concern by stating that hundreds of patients had received TSO® treatment in Europe without reported side-effects and added that if symptoms were to occur the causative agent could be easily treated with antihelminthics.
Heitman et al. (Heitman 2007) published a case report of an 8-year old boy who was treated with TSO® for refractory eosinophilic colitis. After receiving 10 doses of TSO® (titration from 100 to 2000 eggs), lactoferrin decreased from 500 mg/1 to 14.5 mg/1 and his colitis symptoms improved (bloody diarrhea subsided). No adverse events were reported.
Abner et al (Abner 2002) demonstrated that Campylobacter jejuni to be pathogenic in the presence of T. suis in pigs. Shin et al (Shin 2004) reported a case of life-threatening C. jejuni colitis associated with concomitant Trichuris ova in the feces in a Somalian patient. Summers et al (Summers 2006) pointed out that Campylobacter jejuni alone causes a severe life- threatening colitis and it is highly speculative whether coinfection with Trichuris accentuates such infections. Although the abstract of the article reported T.suis ova in the stool, the actual text stated that “microscopy showed Trichuris trichiura larvae”. Trichuris larvae are not normally seen in stool specimens, no adult forms were seen on colonoscopy, and improvement occurred
prior to treatment with mebendazole. If this Somolian immigrant was colonized, it was almost certainly the result of a helminth other than T. suis or the patient may have had strongyloides. We suspect this was an error that the authors failed to address.
When patients have an IBD flare, they will usually develop diarrhea (often bloody diarrhea). Stool is always checked for bacterial pathogens. These patients then receive powerful immune suppressants like high dose steroids, azathioprine, methotrexate, and biologics (e.g anti-TNF). Any of these medications would worsen a Campylobacter jejuni infection. So this concern is nothing new. Co-infection with bacterial pathogens, worsening IBD and use of powerful immune suppressants has not proven to be a significant concern in current medical practice.


Nature’s most powerful probiotic:
Use in Children
There have been no clinical studies with TSO® performed in children. There have been individual case reports in the literature; these are summarized as follow:
Heitman, et. al. published a case report of an 8-year old boy who was treated with TSO® for refractory eosinophilic colitis. After receiving 10 doses of TSO® (titration from 100 to 2000 eggs), lactoferrin decreased from 500 mg/1 to 14.5 mg/1 and the bloody diarrhea subsided. No adverse events were reported.
Kradin, et. al. published a case report of an iatrogenic T. suis infection in a 16-yearold adolescent boy. He received 5 oral doses of 2500 TSO® for the treatment of Crohn’s disease. A cecal biopsy showed fragments of a coiled larvae lying directly beneath the attenuated ileocecal mucosal epithelium and a mature adult trichurid free floating in the lumen. Female worms were not identified in the biopsy specimens of the present case. In this patient the bowel mucosa at all sites, including those not actively infected by T. suis, showed a lymphoplasmatic infiltrate with substantial numbers of eosinophils, consistent with the development of a Th2-mediated response. However, the biopsies also showed moderately active CD.
